Researchers from China have developed a groundbreaking method to convert carbon dioxide (CO2) and methane (CH4) into useful chemicals using only high-energy light, eliminating the need for costly catalysts. This innovative approach utilizes a specialized 28-W ultraviolet light source that emits photons at a precise wavelength of 185 nm, enabling the direct breakdown of these greenhouse gases.
The findings, published on December 14, 2025, in the journal Nature Photonics, highlight a potential solution to two major contributors to climate change. Carbon dioxide and methane together account for nearly 84% of the global temperature rise, significantly impacting ocean chemistry and marine ecosystems. The research team aims to not only reduce emissions but also transform existing atmospheric gases into valuable products, contributing to a circular economy.
Challenges of Greenhouse Gas Conversion
Transforming CO2 and CH4 into useful chemicals poses significant challenges due to their chemically stable nature. Methane features strong carbon-hydrogen (C–H) bonds, while carbon dioxide contains robust carbon-oxygen (C=O) bonds, making them resistant to chemical reactions. Traditional methods for conversion often rely on expensive metal catalysts and require extreme conditions, such as temperatures exceeding 700 °C and high pressures, which can be both energy-intensive and costly.
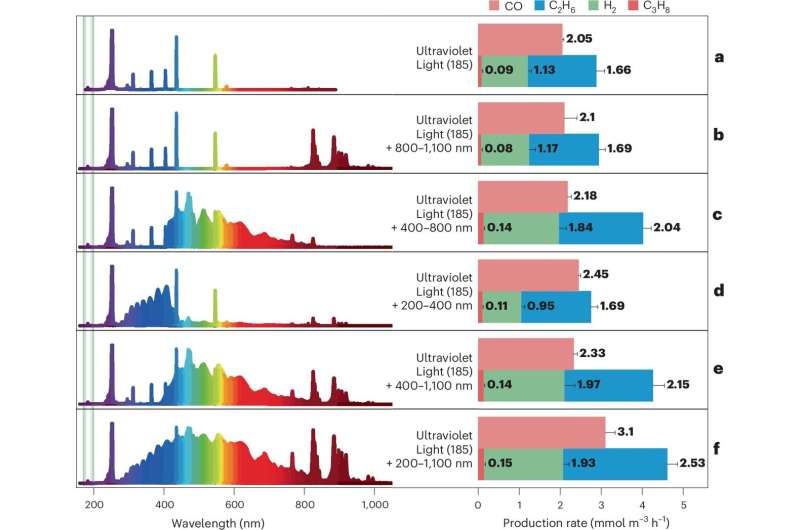
The research team found that high-energy photons could provide the necessary energy to break these strong bonds. They constructed a quartz reactor chamber filled with a mixture of 99.9% pure CO2 and CH4. The chamber was subjected to various light types under controlled conditions, with a temperature set at 25°C. The specific wavelength of 185 nm from the ultraviolet light source, combined with additional light in the 200–1,100 nm range, activated the gas molecules, initiating the conversion process.
Breakthrough Results and Future Implications
The results from gas analysis revealed the production of key chemicals: carbon monoxide, hydrogen, and ethane, with production rates of 3.1 mmol m-3 h-1, 1.93 mmol m-3 h-1, and 2.53 mmol m-3 h-1, respectively. Notably, the researchers observed that incorporating water into the mixture and removing atmospheric oxygen enhanced the yields. In experiments simulating conditions akin to outer space by flushing the chamber with argon gas, they achieved a total gas conversion of 1.51% within 24 hours.
While the yield remains relatively low at this stage, the researchers view their findings as a promising step toward a new method for converting greenhouse gases into valuable products without the need for catalysts or extreme energy inputs. This advancement could pave the way for more sustainable practices in managing greenhouse gas emissions and developing a more resource-efficient economy.
As the impacts of climate change continue to escalate, innovative approaches such as this one may play a crucial role in mitigating its effects and transforming waste into valuable resources. The research team emphasizes the importance of continued exploration in this field to improve yields and expand the range of chemicals that can be produced.
In conclusion, this study represents a significant leap forward in chemical engineering and environmental science, showcasing the potential of light-driven processes to address pressing global challenges.