Researchers at Xiamen University have made a crucial advancement in cancer treatment with the development of a new class of X-ray-sensitizers (XSs). Their findings, published in the journal Engineering, reveal how specific organic pharmaceutical drug intermediates derived from thioxanthone (TX) can be activated using low-dose X-rays to efficiently generate singlet oxygen for targeted cancer therapy.
Traditional radiotherapy typically requires high doses of radiation, often exceeding 50 Gy, which can lead to severe side effects for patients. To address these concerns, clinicians often resort to fractionated radiation therapy, administering smaller doses of less than 2 Gy across multiple sessions. The research team, led by Hongmin Chen, proposes a novel method to enhance the effectiveness of these smaller doses, potentially minimizing adverse effects while maintaining or even improving therapeutic outcomes.
Innovative Mechanism for Cancer Treatment
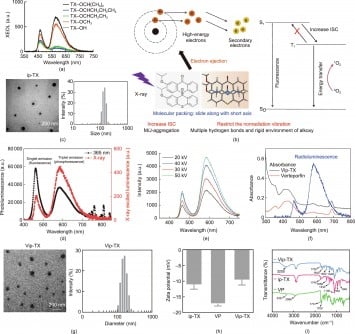
The focus of the research is on TX-derived organic molecules, which display remarkable potential for populating triplet excitons, specifically singlet oxygen, when exposed to X-ray irradiation. This process, termed scintillator X-ray-induced photodynamic therapy, utilizes low-dose X-ray irradiation to activate these molecules, resulting in the selective destruction of cancer cells. This approach offers a more precise and less harmful alternative to conventional radiotherapy.
The team systematically evaluated various pharmaceutical drug intermediates that are derivatives of TX. By modifying the alkoxy side chain substitutions at the 2-position of TX, they were able to fine-tune molecular packing and intermolecular interactions. This meticulous adjustment allowed the researchers to assess the fluorescence and room-temperature phosphorescence (RTP) of these TX derivatives when subjected to X-ray irradiation.
Their findings indicate that TX derivatives exhibit superior radioluminescence compared to phenothiazine compounds with similar substitutions. Notably, these TX-derived molecules demonstrated high efficiency in X-ray-sensitization, generating singlet oxygen in response to low-dose X-ray exposure. This capability is vital for effective cancer treatment, as it means that a large number of triplet states can be populated directly with minimal radiation, potentially reducing the overall radiation dose required for effective therapy.
Promising Future for Cancer Therapy
Extensive evaluations of the molecules’ potential for tumor treatment were conducted both in vitro and in vivo. The ability of TX derivatives to generate singlet oxygen and target tumors emphasizes a promising new pathway for cancer therapy. This breakthrough could lead to the creation of a new class of organic molecules specifically designed for low-dose X-ray radiotherapy, enhancing treatment efficacy while minimizing side effects.
With continued research and development, these TX-derived XSs could become integral to advanced cancer therapies, providing a more effective and patient-friendly alternative to current radiotherapy methods. The implications of this work extend beyond the laboratory, potentially transforming how cancer is treated globally.
The study titled “X-Ray-Sensitizers: Organic Pharmaceutical Drug Intermediates Activated Directly by X-Rays to Efficiently Populate Triplet Excitons for Cancer Treatment” was authored by Nuo Lin, Han Xu, Haichao Liu, Xiaoqian Ma, Qunying Shi, Qing Yang, Yating Wen, Huanglei Wei, Ke Hu, Bing Yang, and Hongmin Chen. The full text of the open access paper can be found at: https://doi.org/10.1016/j.eng.2024.06.010. For updates on research published in Engineering, follow them on X and Facebook.