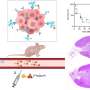
Research published in the November 2025 issue of the Journal of Nuclear Medicine reveals a promising new radioimmunotherapy approach for treating human epidermal growth factor receptor 2 (HER2)-positive breast cancer. This innovative regimen pre-treats tumors before administering targeted alpha-radioimmunotherapy, leading to significant and durable responses with minimal toxicity. The findings suggest a potential breakthrough in creating safer and more effective treatment options for patients facing this aggressive form of breast cancer.
HER2-positive breast cancer represents a challenging diagnosis, with poor prognosis rates, particularly for advanced metastatic cases. Approximately 15–20% of breast cancers overexpress the HER2 oncogene, which has become a recognized therapeutic target. Although existing HER2-targeted therapies have improved patient outcomes, issues such as treatment-related adverse events and tumor resistance remain prevalent.
Dr. Sarah Cheal, an assistant professor at Weill Cornell Medicine in New York, highlighted the concerns surrounding previous studies on HER2-targeted radioimmunotherapy. The use of the alpha-particle-emitting radionuclide 225 Ac had shown effectiveness but was associated with significant toxicity due to alpha-particles being retained in the body. “In our study, we used a pretargeted radioimmunotherapy (PRIT) approach to directly treat the tumor and prevent potent alpha-particles from being absorbed in healthy tissues,” Dr. Cheal stated.
Details of the Study
The research team implemented a three-step intravenous regimen consisting of a bispecific anti-HER2/anti-DOTA antibody, followed by a clearing agent, and concluded with 225 Ac-Pr radioimmunotherapy. The team began by assessing the effects of 225 Ac-Pr dosing on tumor-targeting efficiency and tissue biodistribution within a BT-474 breast cancer xenograft model.
In their evaluations, mice bearing either the BT-474 xenograft or a patient-derived xenograft were treated with one or two cycles of 225 Ac-PRIT, separated by one week. A dose escalation study was also conducted to determine the nephrotoxic absorbed radiation dose. The results were promising; in the BT-474 model, 100% of the mice achieved complete responses, with 85% attaining histologic cures. Both one-cycle and two-cycle treatments demonstrated similar effectiveness, and the treatments were well tolerated with no chronic radiation toxicity noted.
In the patient-derived xenograft model, a single treatment of 225 Ac-PRIT resulted in a 60% complete response rate and extended survival compared to untreated controls. Additionally, researchers identified the dose at which severe chronic nephrotoxicity occurred in the 225 Ac-PRIT regimen.
Implications for Future Treatments
“This study illustrates the curative potential of 225 Ac-PRIT as a treatment for highly aggressive subtypes of HER2-positive breast cancer,” said Dr. Nai Kong Cheung, a member and attending physician in Pediatric Oncology at Memorial Sloan Kettering Cancer Center. He added that if this therapy is successfully translated into clinical practice, it could provide new treatment avenues for breast cancer and other HER2-expressing solid tumors.
The findings from this study signify a notable advancement in the fight against HER2-positive breast cancer, offering hope for more effective and safer treatment options for patients in the future. The research underlines the importance of continued exploration into innovative therapies that could transform the current landscape of breast cancer treatment.
For further details, refer to the original research by Sara S. Rinne et al, “225 Ac α-Pretargeted Radioimmunotherapy of Human Epidermal Growth Factor Receptor 2–Expressing Breast Cancer,” published in the Journal of Nuclear Medicine.