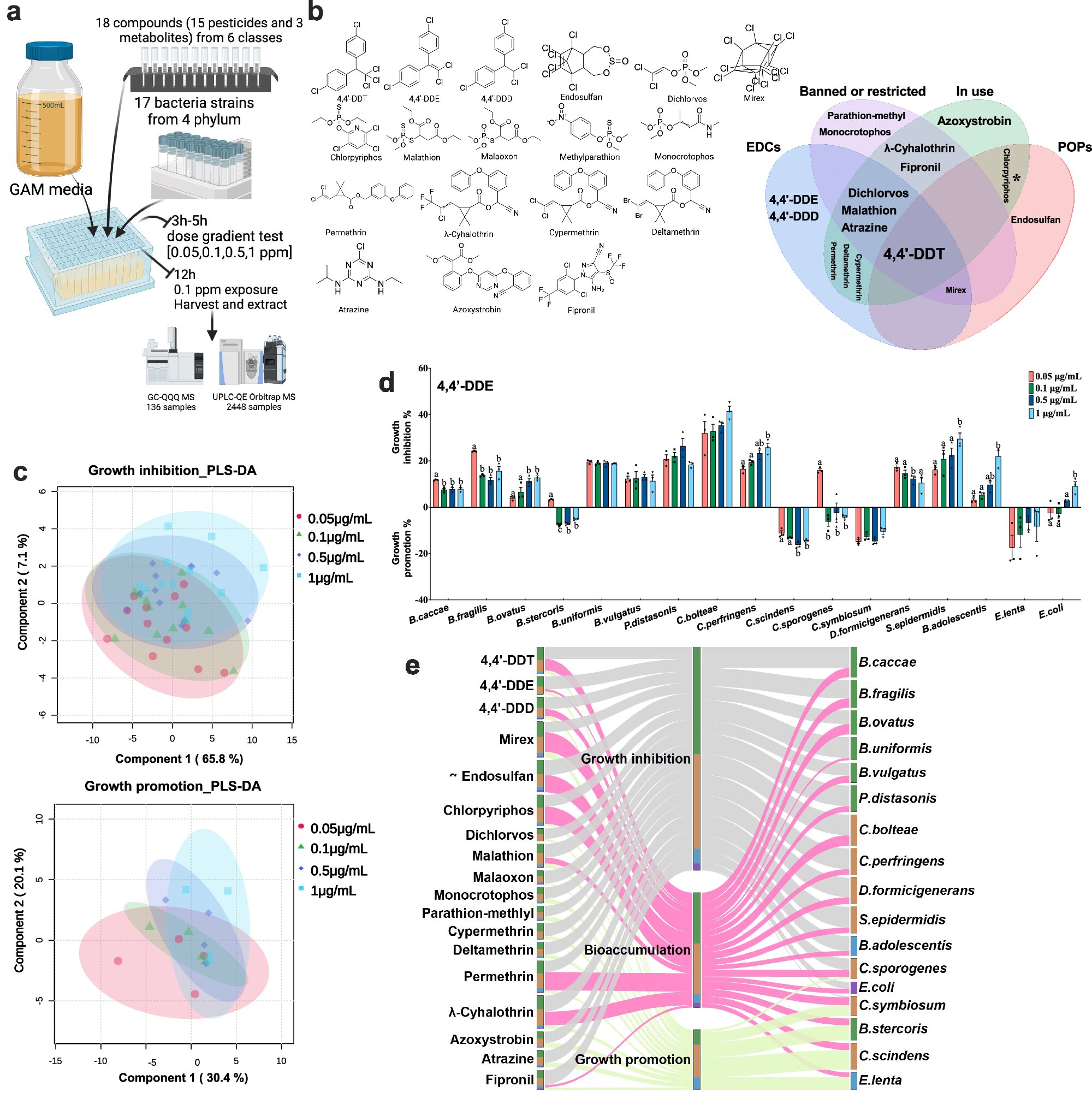
A groundbreaking study has unveiled a detailed atlas illustrating how pesticides affect gut bacteria, potentially paving the way for probiotic interventions. Published in Nature Communications, the research highlights the complex interactions between pesticides and the human microbiome, revealing significant implications for health and disease prevention.
The study, conducted by researchers at The Ohio State University and other institutions, is the first of its kind to map changes in specific gut bacteria due to interactions with insecticides. The findings suggest that over a dozen pesticides can alter the growth patterns of gut microorganisms, affecting how they process nutrients and reside within certain bacteria. This comprehensive atlas of molecular mechanisms is now publicly available, offering a valuable resource for future studies on diseases and therapeutic strategies.
Understanding the Impact of Pesticides on Gut Health
Emerging evidence has long suggested that pesticides may be toxic to the diverse microorganisms residing in the human digestive system. However, this new study provides the first detailed map of how specific gut bacteria are affected by pesticide exposure. The research involved 18 widely used pesticide compounds and 17 species from four major bacterial domains in the human gut, associated with health maintenance or disease states.
Among the pesticides examined were DDT, atrazine, permethrin, and chlorpyrifos. Despite restrictions on their use, residues from some legacy pesticides continue to persist in soil and water, posing ongoing risks to human health. “We grew bacteria in culture and exposed them to relevant concentrations of pesticides to observe microbial responses,” explained Li Chen, the study’s first author and a senior research associate in Jiangjiang Zhu’s lab at Ohio State.
Key Findings and Potential Probiotic Interventions
The research revealed that certain gut bacteria species might offer protection against pesticide toxicity, hinting at the potential for probiotic approaches to mitigate health impacts such as inflammation. “We’ve provided further understanding of how pesticides or environmental pollutants impact human health by modulating an important collection of microorganisms,” said senior author Jiangjiang Zhu, an associate professor of human sciences at The Ohio State University.
Experiments conducted on mice demonstrated that introducing Bacteroides ovatus, a common human gut bacterium, could buffer against inflammation induced by pesticide exposure. This finding suggests that specific microbes could be harnessed as therapeutic agents to help clear pesticide-induced toxicity from the gut.
Mapping the Network of Bacteria-Pesticide Interactions
The study’s comprehensive analysis identified 306 pesticide-gut microbe pairs, detailing how these interactions alter metabolic processes. The researchers developed a bacteria-pesticide interaction network, highlighting which pesticides promote or inhibit bacterial growth and which bacteria absorb pesticide chemicals. This network provides crucial insights into how pesticide exposure can be prolonged within the body.
“Most previous environmental health studies reported that pesticide contamination affects the overall composition of gut bacteria,” said Li Chen. “We showed those pesticides really can affect specific gut bacteria and detailed how these changes will affect the general composition.”
Moreover, the study examined changes in metabolites—the molecular products of biochemical reactions crucial for energy production and other functions. The team also focused on lipids, essential compounds produced by gut microbes, revealing that pesticide exposure could alter lipid production and metabolic activity.
Future Directions and Implications for Health
The implications of this research are vast, offering new avenues for understanding and mitigating the health effects of pesticide exposure. The study’s findings provide a foundation for further exploration of how metabolic changes in gut microbes relate to various health and disease conditions following pesticide exposure.
“We are mapping out this central interaction between pesticides and gut microbes,” Zhu explained. “Other labs can leverage what we have discovered—for example, after exposure to a pesticide, gut microbe reactions may lead to downstream consequences that contribute to disease research and eventually help with predicting targets or identifying an intervention strategy.”
As the research community continues to explore these interactions, the potential for developing targeted probiotic therapies to combat pesticide-induced health issues becomes increasingly promising. The study’s authors, including collaborators from Yale University, Zhejiang Academy in Hangzhou, China, and Johns Hopkins University, anticipate further investigations into these critical interactions.
For more information, the full study can be accessed in Nature Communications under the title “Mapping pesticide-induced metabolic alterations in human gut bacteria” by Li Chen et al.