Lunai Bioworks, an AI-driven biotechnology company based in Sacramento, has identified three clinically relevant subtypes of Parkinson’s disease along with prioritized drug targets. This development aims to enhance proof-of-concept programs and foster strategic partnerships in a market projected to reach $13 billion by the 2030s. The announcement was made on December 9, 2025, and is a significant step in addressing the challenges faced in Parkinson’s therapy development.
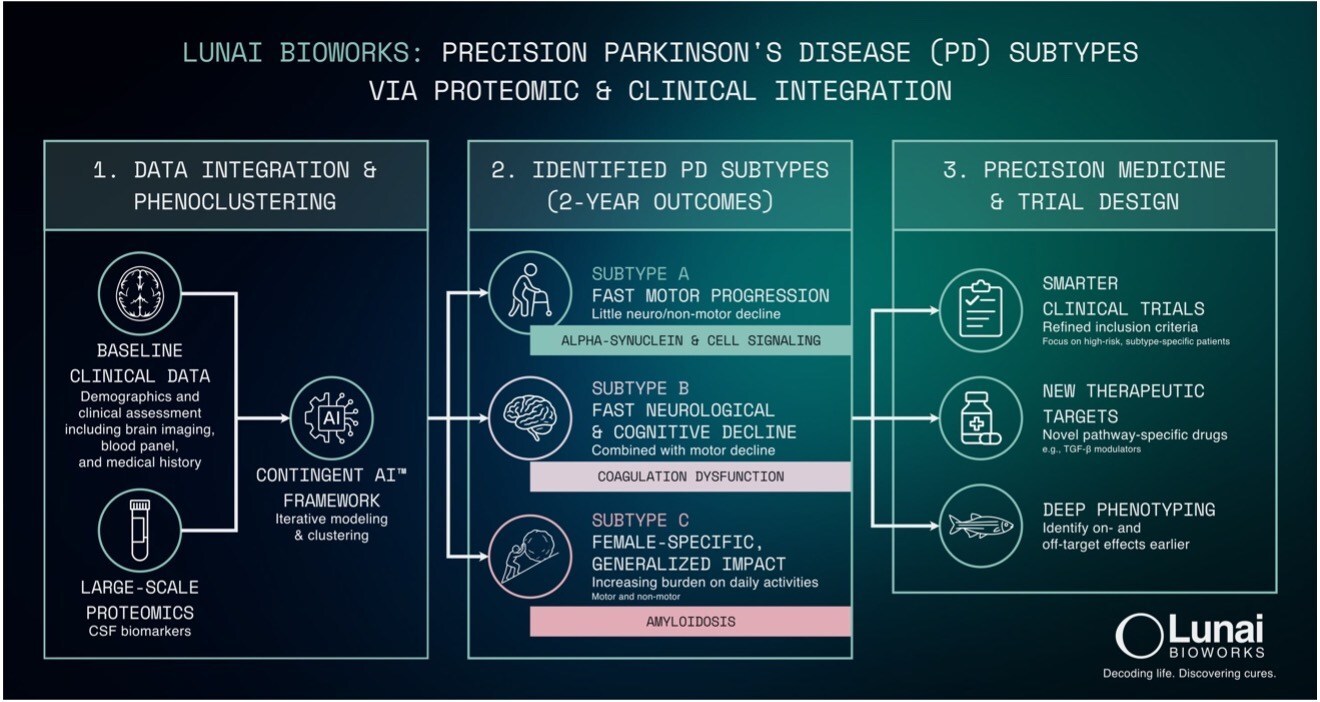
Utilizing its proprietary Augusta Platform, Lunai Bioworks, through its subsidiary BioSymetrics, analyzed extensive proteomic data from the Parkinson’s Progression Markers Initiative (PPMI). This landmark study, overseen by the Michael J. Fox Foundation, tracks thousands of patients over time to identify biological markers associated with disease progression. The analysis integrated longitudinal clinical data from over 650 participants, examining 4,500 proteomic probes over several years, with a median tracking period of at least 2.5 years.
The integration of this data allowed for the statistically robust identification of molecular signatures linked to faster disease progression and poorer outcomes. Such findings are critical for improving patient selection, accelerating proof-of-concept trials, and increasing the chances of clinical and commercial success for therapeutics targeting specific subtypes.
Three distinct patient subtypes were identified, each linked to specific outcomes. This discovery forms a foundation for more effective trial strategies, aiming to improve success rates and reduce the time needed to achieve proof-of-concept. The evaluation of proteomic data also highlighted targets and biomarker candidates that could assist in baseline stratification, monitoring disease progression, and assessing treatment responses.
Lunai Bioworks is now moving forward with experimental validation of these prioritized targets, progressing towards preclinical model development and the qualification of biomarkers. According to David Weinstein, CEO of Lunai Bioworks, “As Parkinson’s therapy development continues to struggle with high failure rates and slow progression signals, subtype-specific strategies can materially improve outcomes.” He emphasized the potential of linking clinical trajectories to biological pathways to design smarter trials and identify viable targets.
In addition to its internal efforts, Lunai Bioworks is exploring co-development and partnership opportunities with biopharmaceutical companies. These partnerships could involve applying subtype-specific inclusion criteria to existing Parkinson’s assets, co-developing biomarkers and companion diagnostics, and translating insights from biological pathways into innovative therapeutics.
Dr. Gabe Musso, Chief Scientific Officer of BioSymetrics, remarked on the synergy of molecular biology and clinical phenotyping, stating, “Integrating molecular biology with clinical phenotyping gives us a mechanism for identifying precision targets that could reshape how Parkinson’s therapies are developed.” This integration aims to facilitate faster validation processes and more efficient partnership strategies.
The market for Parkinson’s disease therapies is currently valued between $6 billion and $8 billion, with expectations of significant growth driven by rising prevalence and unmet medical needs. Lunai Bioworks is committed to leveraging its advancements in phenomics, proteomics, and precision stratification to increase clinical success rates and support high-value partnerships.
Lunai Bioworks Inc. (NASDAQ: LNAI) is dedicated to pioneering safe and responsible generative biology through AI-powered drug discovery. The company focuses on dual-use risk management while striving to redefine therapeutic innovation and address emerging societal threats. For further information, please visit their website: https://lunaibioworks.com.
This announcement highlights the potential for transformative advancements in the treatment of Parkinson’s disease, as Lunai Bioworks positions itself at the forefront of this evolving field.