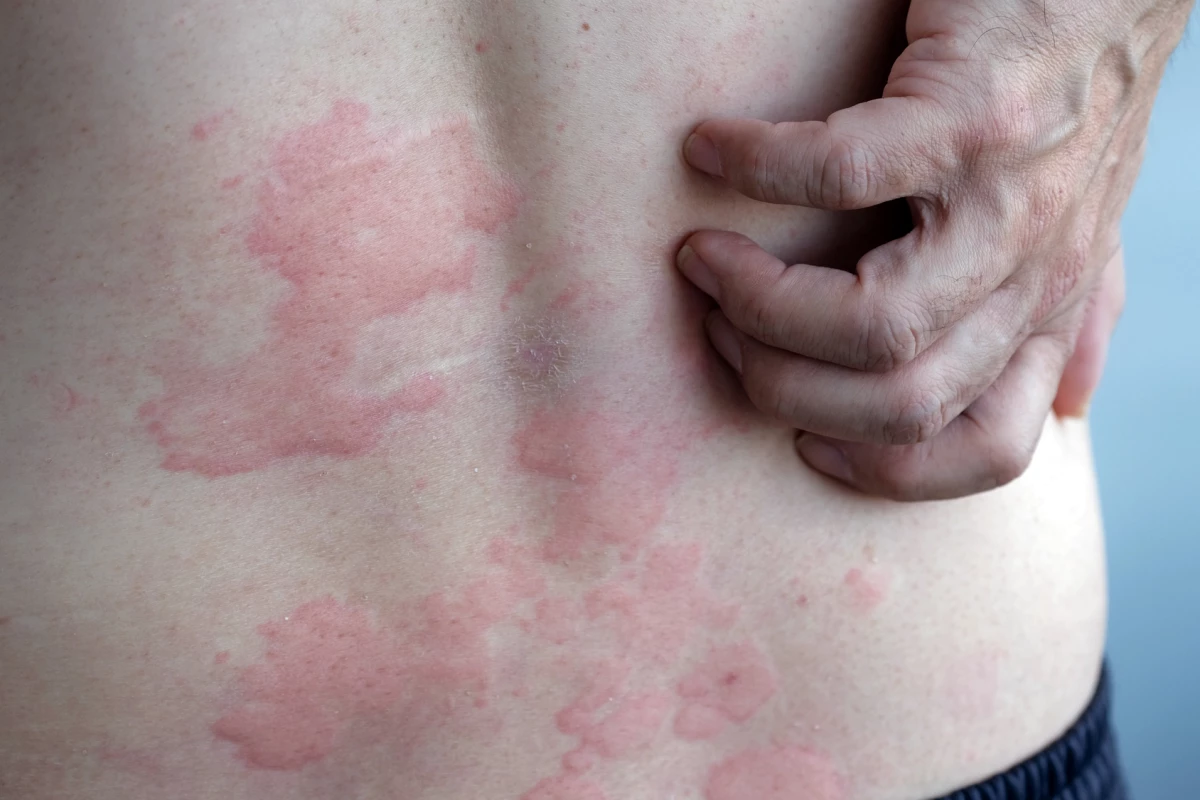
A comprehensive global study has established a hierarchy of effective treatments for chronic hives, also known as urticaria, particularly when standard antihistamines are ineffective. Led by researchers at McMaster University in Canada, this large-scale investigation offers valuable insights for both patients and healthcare providers.
Chronic hives manifests through intense itching, swelling, and red welts on the skin. A diagnosis of chronic urticaria is made when these symptoms persist for more than six weeks. While antihistamines are typically the first-line treatment, this new study identifies alternative systemic therapies that can provide relief when antihistamines do not suffice.
Study Overview and Methodology
The study, which analyzed data from 93 studies involving 11,398 participants, primarily focused on adolescents and adults suffering from moderate to severe chronic urticaria symptoms. Researchers employed a systematic review and a Bayesian network meta-analysis (BNMA) to evaluate the safety and effectiveness of various drugs and immunotherapies specifically for chronic urticaria. Only randomized controlled trials for systemic treatments were included, while studies on antihistamines, steroids, and alternative therapies were excluded.
According to Derek Chu, MD, PhD, the corresponding author and assistant professor in McMaster’s Department of Medicine, “This first comprehensive analysis of all advanced treatment options for chronic urticaria provides a clear and evidence-based ‘menu of treatment options’ for patients and their clinicians to choose from.”
The study evaluated key outcomes such as urticaria activity, severity of angioedema (swelling), quality of life, and adverse events related to treatments. Each trial was rigorously reviewed for quality and risk of bias, leading to a high certainty of evidence for several treatment options.
Top Treatment Options Identified
The analysis ranked the most effective treatments, with a high level of certainty regarding their benefits. The leading options included omalizumab (Xolair), administered at a dosage of 300 mg every four weeks, and remibrutinib. Omalizumab is an injectable monoclonal antibody that blocks immunoglobulin E (IgE), a key player in allergic reactions and inflammation. Remibrutinib, although not yet commercially available, has shown promising results in Phase 3 clinical trials, targeting Bruton’s tyrosine kinase (BTK) to inhibit the release of histamine and other inflammatory mediators.
Another significant treatment identified was dupilumab (Dupixent), which blocks interleukin-4 (IL-4) and interleukin-13 (IL-13), two proteins involved in inflammation. While the research indicated its likely effectiveness in reducing itch and wheal severity, the impact on angioedema and overall quality of life remained uncertain due to insufficient data.
The study also evaluated cyclosporine, an immunosuppressive medication that can effectively reduce itch and wheals. However, it was associated with higher rates of adverse effects, including kidney toxicity and elevated blood pressure.
Despite its strengths, the study acknowledged certain limitations. Many trials were of short duration, making it challenging to assess the long-term safety of some drugs. Additionally, older and less expensive medications, such as sulfasalazine, azathioprine, and methotrexate, were found to have low certainty of evidence, primarily due to small or non-randomized studies. Pediatric data were also limited, with only one trial involving participants under the age of 12.
The study, funded by the American Academy of Allergy, Asthma and Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI), has been published in The Journal of Allergy and Clinical Immunology. It represents the most extensive evidence-based comparison to date of systemic treatments for chronic urticaria, offering a clearer treatment roadmap for patients and clinicians.
As healthcare providers consider these findings, practical aspects such as treatment costs, administration methods, and patient preferences will play crucial roles in developing personalized treatment plans.