On November 14, 1985, a groundbreaking discovery emerged from the laboratories of Rice University in Houston, Texas: the identification of buckyballs, a new class of molecules exhibiting remarkable symmetry. This achievement was the culmination of intense research conducted by chemists Harry Kroto, Richard Smalley, and Robert Curl, who sought to understand the composition of organic molecules in interstellar space.
The journey to this discovery began in the 1970s when Kroto, then a chemist at the University of Sussex in the U.K., was intrigued by the presence of organic molecules in the vast dark clouds between stars. His inquiry was motivated by radio and light data suggesting an abundance of long carbon chains that contradicted existing astrophysical theories. This prompted scientists to consider whether cooling red giant stars might be enriching the interstellar medium with these carbon structures.
Kroto’s pivotal moment arrived during a visit to Rice University in September 1985, where he collaborated with Smalley and Curl. The team utilized a unique apparatus that Smalley had developed, which involved vaporizing metal atoms with a laser beam. By replacing the metal disk with a graphite disk, they aimed to replicate the conditions of red giant stars. Over a span of ten days, the researchers successfully generated six-to-eight-carbon chains, further supporting their hypothesis.
Unexpectedly, they also detected larger carbon structures, specifically molecules composed of 60 and 70 carbon atoms. While a similar compound had been noted in an earlier experiment at the Exxon Corporate Research Science Laboratory, it had not garnered significant attention. Kroto referred to these findings as “uninvited guests,” yet they would prove to be the foundation of a monumental scientific breakthrough.
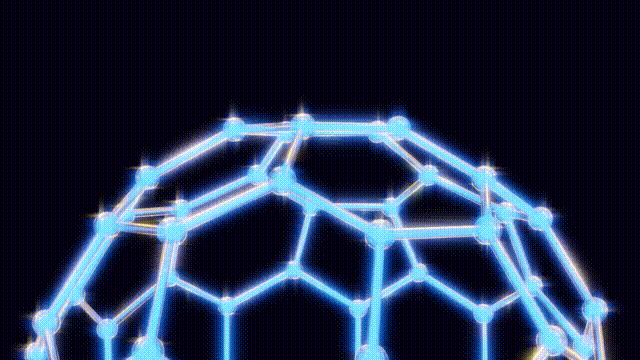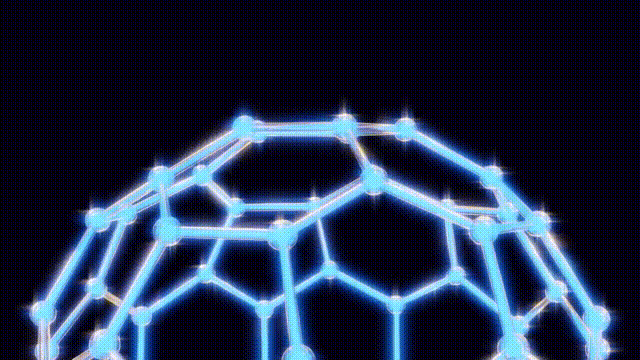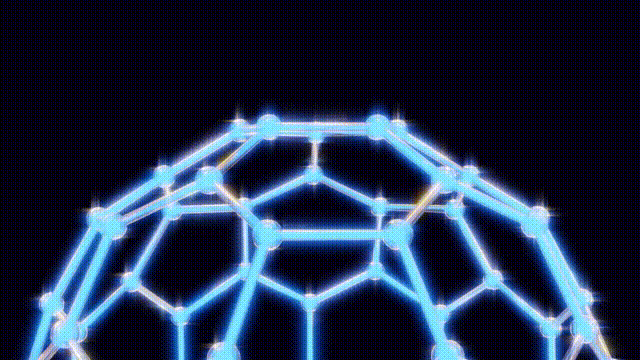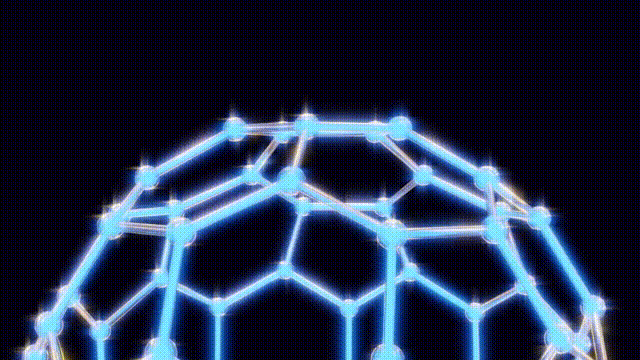
On September 9, after extensive modeling using toothpicks and jellybeans to visualize molecular structures, the team concluded that the 60-carbon molecule, later named buckminster fullerene, defied initial expectations. Kroto recalled an experience from the 1967 Expo in Montreal, where he had seen the geodesic dome, a design created by futurist Buckminster Fuller. This inspiration led them to recognize the spherical structure of buckyballs, which are characterized by pentagonal and hexagonal arrangements of carbon atoms.
The findings were formally published in the journal Nature on November 14, 1985, leading to the popular nickname “buckyballs.” Over the following years, researchers explored the properties of fullerenes, a class of molecules that includes buckyballs. By 1990, scientists had developed methods to create these structures in bulk, utilizing electric arcs between carbon rods.
Kroto, Smalley, and Curl were awarded the Nobel Prize in Chemistry in 1996 for their pioneering work on buckyballs. The implications of their discovery extend far beyond theoretical chemistry. Fullerenes have shown potential in various applications, while their chemical relatives, known as nanotubes, exhibit extraordinary strength and exceptional electrical and thermal conductivity. These nanotubes have become essential in industries ranging from electronics to materials science, featuring prominently in atomic force microscopes, batteries, and biosensors.
Despite the promising potential of buckyballs in areas such as quantum computing and drug delivery, the search for mainstream applications continues. The legacy of Kroto, Smalley, and Curl’s discovery remains a testament to the profound impact that innovative scientific inquiry can have on our understanding of molecular structures and their applications in the modern world.