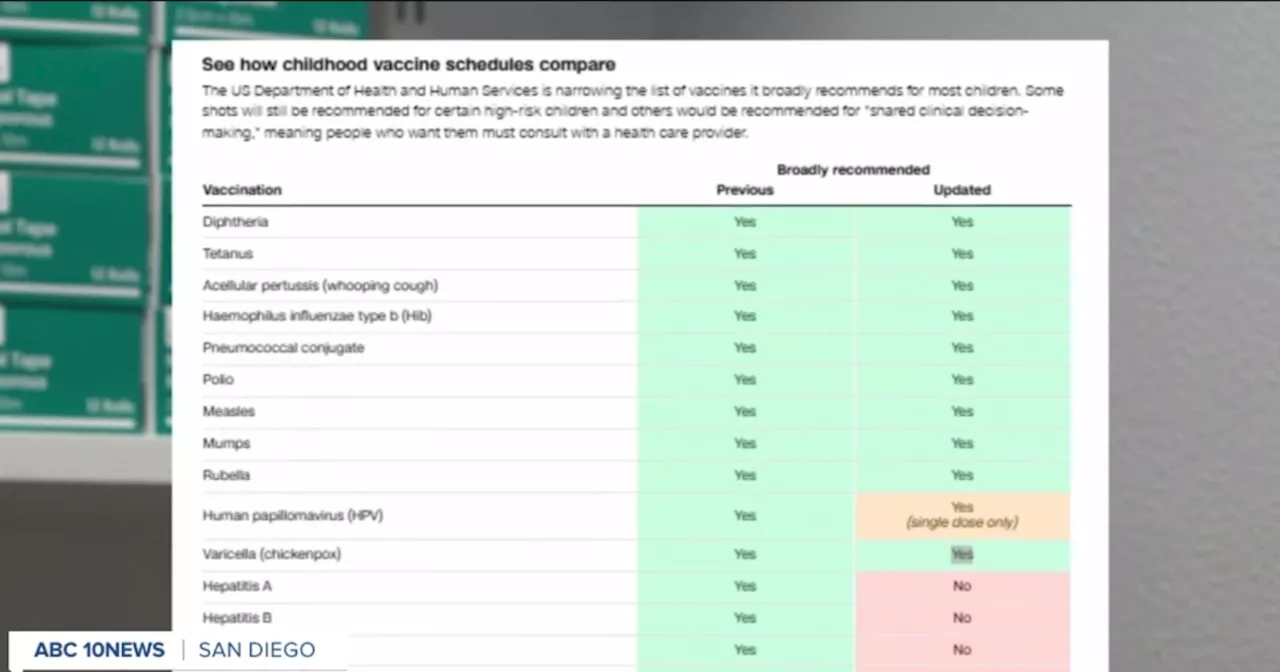
The Centers for Disease Control and Prevention (CDC) has introduced a new federal vaccine schedule for children, reducing the recommended vaccines from 17 to 11 for healthy children. This significant change has raised concerns among pediatricians, educators, and parents regarding the potential impact on public health.
Dr. John Bradley, Medical Director of Infectious Diseases at Rady Children’s Hospital, expressed his discontent with the decision-making process. For years, the American Academy of Pediatrics (AAP) has collaborated with the CDC to shape national vaccine guidance, focusing on scientific evidence, vaccine safety, and effectiveness. Dr. Bradley noted, “Everyone contributes, Everyone has a chance to raise their hand and say, ‘What about this? What about that?’”
The recent updates, released this week, have diverged from the traditional collaborative approach. Instead of consulting with the AAP, the CDC announced its recommendations without prior discussion. This has prompted a backlash from the AAP, whose president described the changes as “dangerous and unnecessary.”
Dr. Bradley emphasized the risks associated with the new guidance. “It’s dangerous because if you don’t give vaccines that we’ve been giving and have been shown to be safe and effective, and a child ends up in the hospital, that’s preventable,” he said. The change in recommendations could lead to complications in vaccine access and insurance coverage, leaving parents and healthcare providers navigating uncharted waters.
In light of this revised schedule, many pediatricians are concerned about the potential confusion it may create among families. “If we can’t recommend it in association with the CDC any longer, that takes away some of the credibility the pediatricians may have,” Dr. Bradley stated. Such uncertainty could undermine trust in medical guidance and affect vaccine adherence.
With the new schedule indicating a shift in recommendations for certain vaccines to high-risk groups, questions remain about how these changes will be implemented in practice. The AAP and various professional societies have voiced their hopes that the CDC will reconsider its approach and engage in discussions with stakeholders before making significant policy changes in the future.
Dr. Bradley remains optimistic but cautious. “I hope that they learn that this pushback from all the professional societies and parent groups will have them think twice about just releasing a policy for vaccines without getting everyone’s input,” he remarked.
As parents and pediatricians work to understand the implications of this new guidance, the conversation surrounding childhood vaccination continues to evolve, highlighting the need for clear communication and collaboration in safeguarding public health.