Cardiff Oncology, Inc. has announced encouraging results from its ongoing Phase 2 clinical trial, CRDF-004, which evaluates the efficacy of onvansertib in combination with standard-of-care treatments for patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). The trial demonstrated a 49% confirmed objective response rate (ORR) in patients receiving a 30 mg dose of onvansertib, compared to a 30% ORR in the control group. This data reflects the intent-to-treat population of 110 patients, with results based on a blinded, independent central review of tumor scans as of July 8, 2025.
Dr. Roger Sidhu, Chief Medical Officer of Cardiff Oncology, expressed optimism about the findings, noting a 19% improvement in confirmed ORR, alongside a quicker response time and deeper tumor regression with onvansertib combined with standard treatments. “Early progression-free survival (PFS) data show a trend favoring the 30 mg dose of onvansertib compared to the control,” he stated. This trial builds upon previous findings released in December 2024, indicating that onvansertib could represent a significant advancement in treating first-line RAS-mutated mCRC.
Trial Design and Efficacy Data
The CRDF-004 trial enrolled patients with mCRC exhibiting documented KRAS or NRAS mutations. Participants were randomized into several groups, including those receiving 20 mg or 30 mg of onvansertib in combination with standard therapies such as FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. The primary endpoint of the study is the objective response rate, with secondary endpoints including progression-free survival, duration of response, and safety metrics.
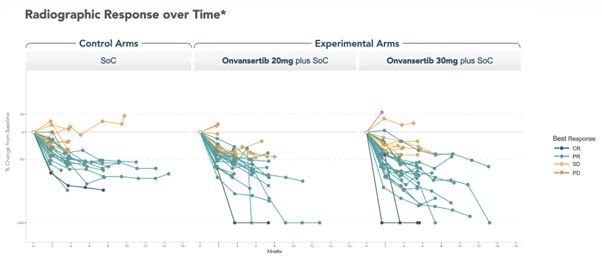
Efficacy results from the trial indicated significant improvements in the 30 mg dose group, which also showed deeper responses compared to both the control arm and the 20 mg dose group. Spider plots detailing changes in tumor size revealed notable reductions in patients treated with the higher dosage.
Both dose arms exhibited early separation in the PFS curves compared to the control group, suggesting a dose-dependent effect favoring the 30 mg onvansertib cohort. While the median PFS has yet to be reached, the initial data is promising.
Safety and Future Prospects
Safety assessments were conducted on 104 patients who received treatment during the trial. Notably, onvansertib was well-tolerated, with no major or unexpected toxicities reported. The most common adverse event associated with onvansertib was neutropenia, which occurred infrequently.
Dr. Mark Erlander, Chief Executive Officer of Cardiff Oncology, highlighted the study’s successes, stating, “The strength of our data achieves the key objectives we set for the trial, positioning us to engage in discussions with the FDA as we advance toward our registrational CRDF-005 trial.” He expressed optimism regarding onvansertib’s potential to redefine the first-line treatment landscape for RAS-mutated mCRC and announced plans for an update on the program by Q1 2026.
The company will host a conference call today at 4:30 p.m. ET / 1:30 p.m. PT, inviting interested parties to participate through a webcast available on its website. A replay will also be accessible in the investor relations section post-call.
Cardiff Oncology continues to focus on leveraging PLK1 inhibition to develop innovative cancer therapies. The company’s lead asset, onvansertib, shows promise not only in mCRC but also in trials targeting other cancer types, including metastatic pancreatic ductal adenocarcinoma and triple-negative breast cancer.
For more information, please visit [Cardiff Oncology’s website](https://www.cardiffoncology.com).