Recent research has revealed that cancer cells can influence neighboring healthy cells by transferring their mitochondria, the energy-producing components of cells. This process allows tumors to essentially “reprogram” adjacent cells to support cancer growth. The study, published on August 28, 2023, in the journal Nature Cancer, was led by Sabine Werner and her team at ETH Zurich.
For years, it has been established that cancer cells can hijack mitochondria from surrounding cells. However, this new investigation indicates that the reverse is also true; cancer cells can send their mitochondria into neighboring healthy cells, a phenomenon not previously documented. Werner emphasizes that this reciprocal transfer of mitochondria is critical for understanding how cancer spreads and evolves.
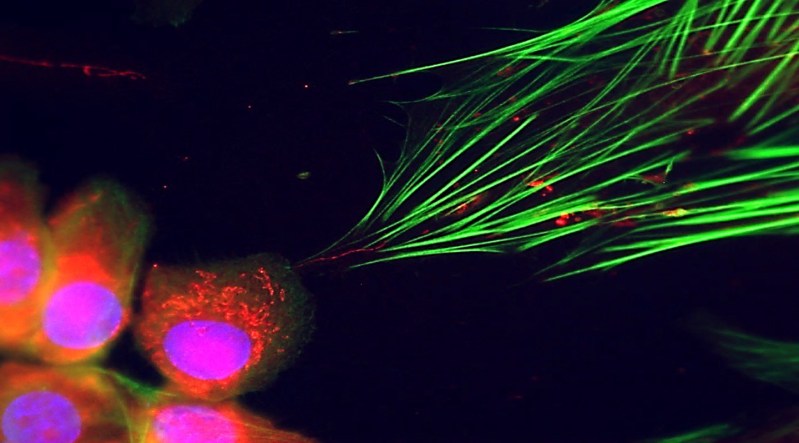
During their research, Werner’s team initially aimed to explore the communication between cancer cells and fibroblasts, a type of connective tissue cell. Their investigation took an unexpected turn when a colleague noticed a peculiar structure—what appeared to be a tunneling nanotube connecting the cancer cells to fibroblasts. This structure facilitates the movement of mitochondria and other cellular materials between cells.
In the laboratory, the team observed that mitochondria from cancer cells were indeed entering fibroblasts. This influx resulted in the fibroblasts becoming more active, leading to accelerated growth and increased expression of cancer-associated genes. The researchers noted that within just 24 hours, these mitochondria-rich fibroblasts began to exhibit characteristics of cancer-supporting cells. When these altered fibroblasts were injected alongside cancer cells into mouse models, they contributed to tumor development.
The study identified a specific protein, MIRO2, as essential for this mitochondrial transfer process. MIRO2 facilitates the transport of mitochondria to the cell membrane, where the nanotubes form. Without this protein, cancer cells struggle to deliver their mitochondria to neighboring fibroblasts, highlighting its potential as a therapeutic target.
The implications of this research extend beyond fibroblasts. According to findings presented earlier this year by Yosuke Togashi from Okayama University, cancer cells can also transfer mitochondria to immune cells. This transfer appears to diminish the immune cells’ ability to combat cancer, further complicating the body’s response to tumors.
Mitochondrial transfer as a mechanism in cancer biology is an emerging field, and the findings from Werner’s team are paving the way for future inquiries. Jiří Neužil of the Czech Academy of Sciences noted that while the field is still developing, there is strong evidence supporting the notion that cancer cells utilize mitochondria to manipulate their environment.
The study has prompted numerous questions regarding the driving forces, triggers, and mechanisms behind mitochondrial transfer. Werner acknowledges that these findings exemplify the importance of investigating unexpected results in scientific research. The discovery of these cellular structures has opened new avenues for understanding cancer biology and developing innovative therapies aimed at disrupting these processes.
As researchers continue to explore this fascinating area, the hope is that unraveling the complexities of mitochondrial transfer will lead to new strategies in the fight against cancer.